Current Research Studies
Clinical trials and research studies help to develop new interventions and tests that may improve your health care, alleviate the symptoms of your disease or condition or improve the health care of others with the same condition.
The following clinical trials and research studies are currently recruiting participants. Contact the researchers directly if you would like to be involved.
Early Intervention after Knee Replacement Study (EPIK)
Researchers at the University of Sydney and University of New South Wales are developing a model of care for patients with persistent knee pain after total knee replacement. You are eligible if you have currently, or previously experienced persistent knee pain 3 months post total knee replacement.
They are interested in eligible patients’ to participate in the project, drawing on your experience to provide feedback on the proposed model of care before it is implemented in the trial, whether it is positive or negative.
The co-design process includes two interactive, virtual group-based workshops where you will collaborate with researchers and other healthcare professionals to identify key priorities and contribute to the model’s development.
📅 Workshop 1: 10am (AEST) Friday 8th August 2025
📅 Workshop 2: 9am (AEST) Friday 29th August 2025
Via: Virtual Zoom Meeting
If you would like to participate, please review the participant information statement (below) and then email epik.study@sydney.edu.au.
If you have any questions, please do not hesitate to call Dr Giovanni Ferreira (lead investigator) on 02 8627 6691 or email at epik.study@sydney.edu.au.
Psoriatic arthritis clinical trial
Learn about ICONIC-PsA 2 research study of an oral investigational medication for adults who have taken a biologic medication for active psoriatic arthritis (PsA).
This study is evaluating an oral investigational medication for adults who have taken a biologic medication for active psoriatic arthritis (PsA).
To qualify for this study, you must:
• Be 18 years of age or older
• Have been diagnosed with PsA for at least 3 months prior to beginning this study
• Have at least 3 swollen joints and at least 3 tender joints
• Have active plaque psoriasis or nail changes consistent with psoriasis
• Have previously taken 1 biologic medication for PsA or psoriasis
For further information click on the link below-
Do you live with shoulder osteoarthritis, support someone who does, or treat it clinically?
The University of Queensland want to hear what you think is important to research about shoulder osteoarthritis.
Shoulder osteoarthritis (OA) impacts many Australians and New Zealanders and causes significant pain. Answer their survey to help change this.
To get involved, you must:
• Live with, or care for someone with shoulder osteoarthritis
• Provide healthcare in shoulder osteoarthritis
• Be over 18 years old
• Live in Australia or New Zealand
For further information click on the links below-
Are you living with chronic disease?
Macquarie University Lifespan Health and Wellbeing Research Centre invites you to take part in this research project if you have lived experience with chronic illness. The study aims to understand the relationship between chronic illness, social isolation, and loneliness. You will be asked to complete a short online screening survey to determine if the study is suitable for you. If you proceed, you will be asked to participate in a 30-45 minute online survey.
To be eligible to participate you must:
-Be 60 years and over;
-Be diagnosed with at least one of the nine common physical chronic diseases (i.e., -arthritis, asthma, diabetes, back pain, cancer, chronic kidney disease, chronic obstructive pulmonary disease, osteoporosis, cardiovascular disease);
-Be able to read and write English (to the level of the daily newspaper);
-Reside in Australia;
-Not have a cognitive impairment (e.g., due to dementia or brain injury) or significant psychiatric comorbidity (e.g. psychosis, delirium).
You do not have to be experiencing loneliness or social isolation to participate. For further information click on the link below.
Seeking adults with chronic musculoskeletal pain living in Australia and New Zealand.
Researchers from the University of Sydney want to learn more about how and why some people with chronic musculoskeletal pain choose to engage in certain health and wellness practices, such as mindfulness meditation.
Participate in a 15-minute online survey for a chance to win one of six Prezzee gift vouchers.
For more information please see below.

Invitation to participate in a dry eye study.
Researchers at UNSW are conducting a study to understand the effect of 0.1% ciclosporin An eye drops on immune cells at the surface of the eye in people with dry eyes.
All the study medications are approved by the Therapeutic Goods Administration (TGA) in Australia to treat Dry Eyes.
You are a potential participant for the study if
*You are above 18 years of age
*You have signs and symptoms of or have already been diagnosed with dry eye disease
Participants will be asked to complete dry eye tests and follow-up visits, if they agree to participate.
For further information please click on the link below.
Exploring how perceptions of pain influence physical pain processing.
Researchers at Western Sydney University are looking to explore how pain beliefs and perceptions of individuals with chronic musculoskeletal pain influence the way the body processes pain. This study will help inform the development of treatments.
You can participate if you are:
- Aged between 18-65 years
- Are of Anglo-Australian or Arab background
- Have had musculoskeletal pain for 3 or more months
What will I have to do?
You will be asked to participate in a one off 2-hour session that will include completing demographic questionnaires, participating in a 1-hour focus group and collecting safe, non-invasive sensory tests.
For more information contact Ghufran.alhassani@westernsydney.edu.au or scan the QR code on the information sheet below.

OPTIMISE-Pain Science Education Intervention to Optimise Care for Total Knee Replacement Surgery
The University of South Australia is looking to recruit people with knee osteoarthritis who are thinking about surgery or who have had surgery on their knee.
What will the study involve?
Via an online survey you will be asked a few questions to check that you are eligible to participate and then collect information from you such as your age, biological sex, gender, postcode, country of birth, ethnicity, occupation, your motivation for participating, diagnosis of osteoarthritis, other health diagnoses (e.g. mental health diagnoses, painful conditions, cardiovascular disease, autism, diabetes etc.), presence (and intensity) of knee pain, and past/future joint replacement surgeries.
You will then also be asked your thoughts about osteoarthritis and physical activity, your communication and social interaction styles, and how you are feeling (mood). This will take around 15-20 minutes to complete.
Browse the link below for further information and how to participate.
Nutrition Education Study
Flinders University requests assistance with a research project that aims to improve the nutritional education and support available to older adults with frailty.
We are conducting a series of interviews with older adults who have accessed an online nutrition educational website. Our goal is to gather their perspectives on the website’s content, layout, usefulness, and overall value in managing and preventing frailty through good nutrition.
For more information please see below.

Are you curious about the state of your knee health? Are you concerned about the sounds in your knees?
The University of Sydney are conducting a research study to better understand whether the sounds that knee’s make is related to other aspects of your knee health. They would like to understand whether seeing an Accredited Exercise Physiology (AEP) alters your perceptions of what advice to engage in exercise can do to your overall knee health.
They are looking for people who would like to see an Exercise Physiologist about their knee health and be interested in chatting about the noises they have in their knees.
For more information about this study, please email Prof. Jeanette Thom: kneeclinic.study@sydney.edu.au or click on the link below.
Knee Sounds Participant Information
Examining validating and invalidating adults with chronic pain
The University of Sydney is exploring experiences of invalidating healthcare, that is, where someone’s pain is delegitimised, not taken seriously, or dismissed by a healthcare professional (e.g., “your pain is in your head”) and further understanding in how we can increase validating healthcare for people with chronic pain.
Research has shown that invalidation in healthcare settings is associated with poorer psychosocial outcomes for people with chronic pain symptoms. Subsequently, we are interested in how we can reduce invalidating care and increase validating care for people with chronic pain in Australia.
The study is looking for responses from adults (18+) managing chronic pain who are receiving their healthcare in Australia. The study would involve a 10-15 minute survey and an optional online interview.
For further information or to be involved please click on the link below.
Examining validating and invalidating adults with chronic pain
Do you live with polymyalgia rheumatica (PMR)?
The University of East Anglia (UK) is interested in exploring your experiences of living with a diagnosis of polymyalgia rheumatica (PMR). The purpose of this study is to (a) explore patients ‘lived experience’ of relapse and remission associated with PMR and (b) the clinicians’ perspective about which components are important to define relapse and remission.
You are invited to be involved in this study as your contributions will then inform the development of remission and relapse criteria in PMR that can be used routinely in clinical practice.
This study has important implications for clinical decision-making, treatment management, and evaluation of treatment outcomes which are important and relevant to patients with PMR and the clinicians that treat them.
Information Sheet-People living with PMR.docx
If you would like any further information, please contact Dr Max Yates (Clinical Associate Professor) via email at m.yates@uea.ac.uk
Have you received DMARD treatment?
The Canberra Clinical Phenomics Service is a newly established research laboratory at the Australian National University dedicated to developing new clinical tests for complex immune and inflammatory diseases.
To ensure that the tests are developed with consumers in mind, we are seeking an individual with lived experience of biological DMARD treatment for a position on our Consumer Advisory Board. Please note that EOI’s for this position have now closed.
Please contact The Canberra Clinical Phenomics Service directly if you would like to know more about the project in general.
Euan McNaughton (Operations Manager)
Shoulder Pain Survey for Consumers
Have you ever had shoulder pain that has impacted your activities of daily living?
Monash University is seeking people with lived experience of shoulder pain to help prioritise questions that are most important to address in the development of a ‘living’ clinical practice guideline.
This means the guideline will be continually updated as new information emerges.
Taking part involves completing two short online surveys.
For more information, please see click here or contact Dr Romi Haas- romi.haas@monash.edu
Juvenile Dermatomyositis lived experiences
Massey University seeks parents of children aged 5-12 with Juvenile Dermatomyositis in New Zealand, Australia, and the UK for a study on treatment experiences. The research aims to identify areas needing additional support.
Eligibility:
– Parent of a child 5-12 years old with JDM
– Residing in NZ, Australia, or UK
– Over 18 years old
– Access to strong internet connection
– English-speaking
Participation involves completing a 20-minute online survey about healthcare experiences, with entrants eligible for a draw to win a $20 gift card as a thank you. Additionally, select participants may take part in an optional 1-hour interview via Zoom or phone, for which they will receive a $40 voucher.
The study includes a question for children to answer through writing or drawing.
Click on the below to learn more and apply.
Seeking product testers for an innovative refillable container
| Koor is an innovative refillable food container designed for items like yogurts and purees. Its unique design aims to support individuals facing eating challenges, through its intuitive features that assist with independence.
They are interested in product testing with users living with a disability. They will provide up to two Koors per applicant, in exchange they ask that you use the product and complete a product review survey. Interested? Click on the link below to learn more and get involved.
|
Research priorities to reduce the individual and societal burden of osteoarthritis in Australia
You are invited to participate in a research project being conducted at The Centre for Health, Exercise and Sports Medicine at The University of Melbourne.
”What future research on osteoarthritis do you think is most needed?”
For more information and to check eligibility click on the link below.

Deprescribing Opioids - registered healthcare professionals survey
A research team from The University of Sydney is conducting a study about the resource needs and support registered healthcare professionals require to deprescribe (reduce) opioid analgesics at transitions of care (the movement between different care settings or providers).
You must be currently practicing in Australia to participate and the research involves the completion of a short survey which will take up to 15 minutes.
Further information and the survey links are below.
Do you have knee osteoarthritis?
In partnership with the Queensland University of Technology and Western Sydney University, with funding from Arthritis Australia, patients are invited to participate in a research study which aims to optimise pain relief in knee osteoarthritis.
For more information click on the below.
Still feeling the ache after your total knee replacement?
Researchers at The University of Sydney are interviewing patients to learn about their experience of persistent pain following total knee replacement.
In Australia, 70,000 Total Knee Replacements (TKR) are performed every year. While TKR improves overall pain and function, 1 in 4 patients experience persistent pain after TKR.
Led by University of Sydney and University of New South Wales researchers are developing a model of care for people who have persistent pain after knee replacement surgery – the EPIK model of care. EPIK will identify and treat patients, primarily via telehealth for scalability and accessibility across Australia.
For more information click on the links below-

Are you living with chronic disease?
Macquarie University will be conducting focus groups with consumers to identify possible factors that may impact chronic disease outcomes, social isolation and loneliness from a consumers’ perspective.
The end goal of this research stream is to develop an intervention for adults living with chronic disease that targets social isolation and loneliness.
Click on the link below to learn more.
If you have active flares because of systemic lupus erythematosus, consider participating in the TOPAZ Study for SLE.
The TOPAZ Study is evaluating the safety and efficacy of an investigational medicine, compared to a placebo (an inactive substance that looks like and is administered in the same way as the investigational medicine) in people with active systemic lupus erythematosus (SLE) while taking their prescribed lupus treatment.
For those still experiencing symptoms, even with medication participating in the TOPAZ study may be an option.
The TOPAZ study is for people who are 18 years or older, have been diagnosed with moderate to severe systemic lupus erythematosus (SLE) for at least 6 months, and are currently receiving non-biologic SLE treatment.
Locations to participate in this study:
1. Royal Prince Alfred Hospital, Camperdown NSW 2050
2. Box Hill Hospital, Box Hill VIC 3128
3. Fiona Stanley Hospital, Murdoch WA 6150
If you reside in NSW and are interested in the TOPAZ study, please complete a short survey to check if you might be eligible:
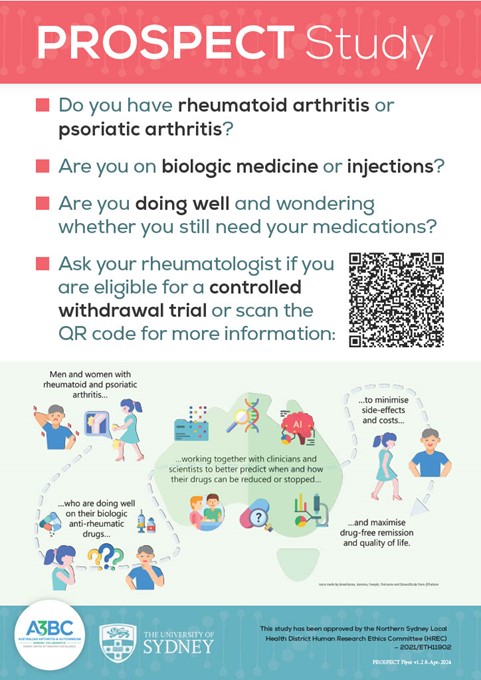
How to safely reduce biologic medications in inflammatory arthritis.
We are looking for people with well-controlled Rheumatoid and Psoriatic Arthritis (RA and PsA) on biologic or targeted-synthetic medications. The trial aims to evaluate the efficacy and cost-saving of safely reducing and stopping these drugs, and through biobank samples for lab analysis you can help us predict a success or failure of drug reduction in the future.
Who can participate?
You may be eligible to participate in this study if you:
- are aged 18 years or over
- first started any biologic or targeted synthetic Disease Modifying Anti-Rheumatic Drugs (b/tsDMARDs) at least 18 months ago
- are in remission or low disease activity and have been stable on DMARD for at least 6 months, and
- if you are on oral corticosteroids, the dose is 5mg or less daily of prednisone or equivalent and has been stable for at least 6 months
- are not on an ineligible DMARD medication (e.g. rituximab, ustekinumab)
- have not had any intravenous (into the blood) or intra-articular (into the joint) corticosteroid injections in the last 6 months
- don’t have concurrent medical conditions that are likely to impact your safety
- are not pregnant
- have not had an investigational new drug within the last 12 weeks
What will you have to do in this study?
You will attend clinic visits in person at a maximum of 8 times in one of our study sites. The dosage will be reduced gradually if you are randomised to a TAPER arm. It will be awesome if you could help with blood and microbiome collections and questionnaires. Travel expenses associated with the visits will be reimbursed, in the form of a gift card with $15 each visit.
Contact us
| State | Trial Site | Principal Investigator | Phone | |
| NSW | Royal North Shore Hospital | Lyn March | jie.yi@sydney.edu.au | 02 9463 1758 |
| St George Hospital | Marissa Lassere | tahi.purni@sydney.edu.au | 02 9463 1893 | |
| Royal Prince Alfred Hospital | Peter Youssef | |||
| SA | The Queen Elizabeth Hospital | Catherine Hill | tuli.tuli@sa.gov.au | 08 8222 7369 |
| Royal Adelaide Hospital | Susanna Proudman | jing.xing@sa.gov.au | 08 7074 2779 | |
| Flinders Medical Centre | Mihir Wechalekar | prospect.trial@sydney.edu.au | 08 8404 4399 | |
| WA | Fiona Stanley Hospital | Helen Keen | fsh.ibdrheumclinicaltrials@health.wa.gov.au (preferred) | 02 9463 1758
08 6151 1154 |
| QLD | Princess Alexandra Hospital | James Gray | fi.thomasresearch@uq.edu.au | 07 3443 6956 |
| Sunshine Coast University Hospital | Matthew Terrill | |||
| VIC | Austin Health | David Liew | victor.yang@austin.org.au | 03 9496 4013 |
| Malvern Rheumatology | Rachelle Buchbinder | prospect.trial@sydney.edu.au | TBC |
Creating a consumer-friendly version of An Australian Living Guideline for the Pharmacological Management of Inflammatory Arthritis
Do you have rheumatoid arthritis, and have you ever wondered what the best course of treatment is? What medications should you be taking, when should they be reviewed, when should you try something new? Monash University researchers are seeking your health with new research that aims to create consumer-friendly resources that are based on new guidelines.
We are conducting interviews (60 minutes) to gauge people’s understanding of the guidelines, what you like about them, how you would improve them, and how we can best implement them in practice.
This research has been approved by the Monash University Human Research Ethics Committee.
To express interest, please contact Dr Danielle Berkovic (danielle.berkovic@monash.edu) at the School of Public Health and Preventive Medicine at Monash University.
Online wellbeing program for young people living with chronic conditions
Online wellbeing program for young people living with chronic conditions
What is it?
The program has three online modules that teach specific skills to help you cope with everyday challenges. Each module takes 60-90 minutes to complete and can be done at your own pace.
Who can take part?
You are eligible to participate if you are:
- Aged 16-25
- Live in Australia
- Diagnosed with a long-term health condition
What’s involved?
If you decide to take part, you will choose one of the three modules to complete and provide feedback on, along with completing four short online surveys over one month. Participants may receive a voucher as reimbursement for their time.
For more information, scan the QR code to read the Participant Information Sheet or email asha.parkinson@telethonkids.org.au
Curtin University Human Research Ethics Committee (HREC) has approved this study (HREC number 2023-0213). The results of this study will be used by Asha Parkinson to obtain a Doctor of Philosophy.

Pain Medicines after total Hip or Knee Replacement
Pain medicines after total hip or knee replacement
We are interested in understanding what people are considering when deciding what to ask for pain medicine when are they discharged from hospital after a total hip or knee replacement.
Who are we looking for?
We would like to engage with men currently scheduled for or on the waiting list for a total hip or knee replacement surgery.
What will you need to do?
Complete a brief online survey asking for your consent to join the study and to collect some basic details about you.
One video or telephone interview (20min to 1 hour) at a time convenient to you (with the possibility of a request for a follow up interview, which you are not obliged to accept).
Link to the survey: https://redcap.sydney.edu.au/surveys/?s=PACCHJMHPY3L7HLX
Contact us
Email us at caitlin.jones@sydney.edu.au if you have any questions or if you would like a copy of the participation statement.

Consumers in Basic Science Research Project
Consumers in Basic Science Research Project
This research project aims to explore consumer involvement in pre-clinical research (research before testing the interventions in people), specifically in the field of musculoskeletal conditions. Consumers, such as people with lived-experience of a musculoskeletal condition and their carers are invited to participate by completing an anonymous online questionnaire. The findings of this study will help understand the current level of consumer involvement and identify opportunities to increase their participation in research.
If you would like to participate in this research, please click on the link below:
https://uniofqueensland.syd1.qualtrics.com/jfe/form/SV_0d0xIWpPgxUZfsW
Participants needed: people with knee arthritis considering keyhole surgery - DECIDE Study - Monash University
Participants needed:
people with knee arthritis considering keyhole surgery
RECRUITING NOW !
Do you have knee arthritis and are considering arthroscopy (also known as a keyhole, cleanout or knee scope procedure)?
Would you like to receive information about osteoarthritis that may help you make better decisions about the care you receive?
If so, you may be eligible to participate in the DECIDE study conducted by Monash University and Cabrini Health. In the DECIDE study, you will be provided with information about osteoarthritis and asked to complete online surveys.
You may eligible if you are:
- 45 years or older
- Diagnosed with knee osteoarthritis
- Considering knee arthroscopy (keyhole surgery)
DECIDE study participants will go into the draw to win a $500 gift voucher. Follow this link to read more and participate in this study: www.decidestudy.com
Alternatively, you can contact the study team using the details below.
Chief Investigator: Dr Denise O’Connor
Email: decidestudy@monash.edu
Phone: 03 9903 8885
Sign up to Arthritis Insights
Regular updates, news and research findings delivered to your inbox: